 The Muscle Immunohistochemistry and Molecular Imaging Core (MIMIC), directed by Dr. Kate Kosmac, offers expertise, training and protocols for researchers working with muscle, including sectioning of frozen muscle tissue, histological and immunohistochemical muscle characterization and imaging of common muscle features. MIMIC provides access to automated image analyses software Myovision for download, and will provide high throughput image quantification upon request.
The Muscle Immunohistochemistry and Molecular Imaging Core (MIMIC), directed by Dr. Kate Kosmac, offers expertise, training and protocols for researchers working with muscle, including sectioning of frozen muscle tissue, histological and immunohistochemical muscle characterization and imaging of common muscle features. MIMIC provides access to automated image analyses software Myovision for download, and will provide high throughput image quantification upon request.
We are proud to support basic, clinical and translation research aimed at understanding the mechanisms underlying the regulation of muscle structure and function and the impact on physical activity and chronic disease. If you have questions or would like to schedule a consultation or reserve time on MIMIC equipment (listed below), please contact us.
MIMIC Techniques and Protocols
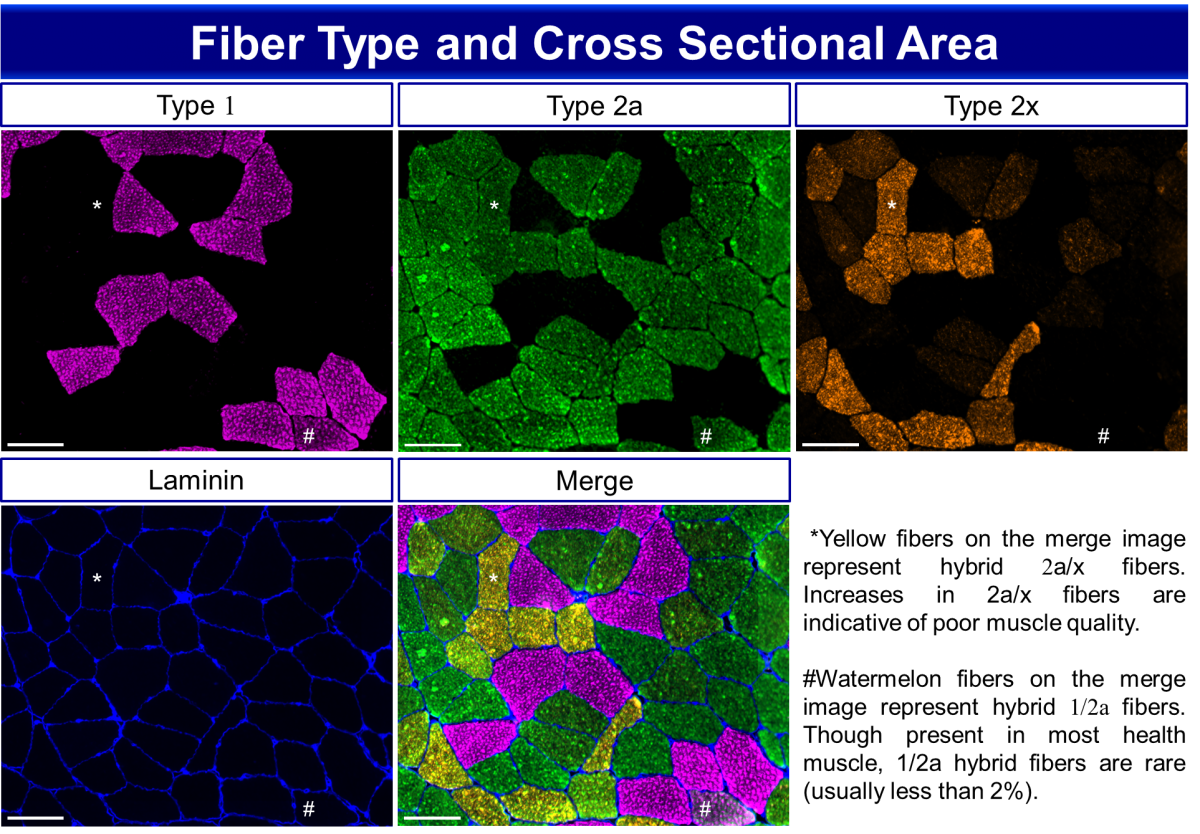
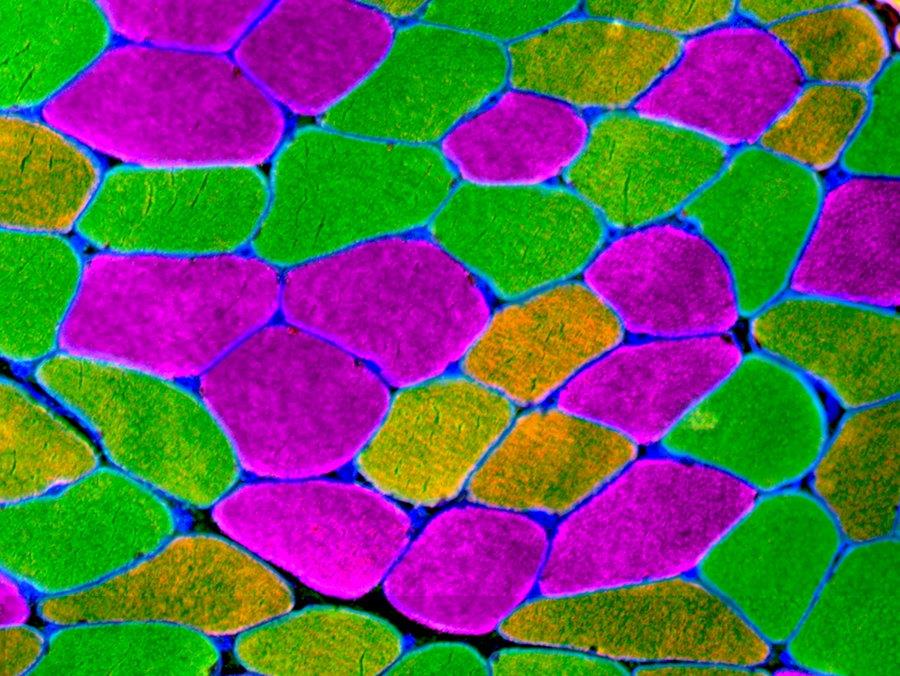
Immunohistochemical analysis of fiber type distribution in the vastus lateralis muscle of a patient with osteoarthritis. Scale bar = 100 µm. Using anti-laminin to label fiber borders, total and fiber type specific cross sectional area can be analyzed. Human skeletal muscle is composed of three myosin heavy chain (MyHC) isoforms: 1, 2a and 2x, identified by isoform-specific antibodies. Fiber type composition is one factor underlying the contractile and metabolic properties of a muscle. Hybrid fibers, composed of more than one MyHC, can also be found in human skeletal muscle. MyHC 1/2a fibers are present but rare, typically comprising less than 5% of total fibers in a biopsy. Hybrid 2a/2x fibers are more commonly observed, whereas pure 2x fibers are also rare.
Fiber Type Staining on Human Muscle Cryosections
Mouse skeletal muscle expresses 4 MyHCs, including 2b, which can be quantified using a modified immunohistochemistry protocol.
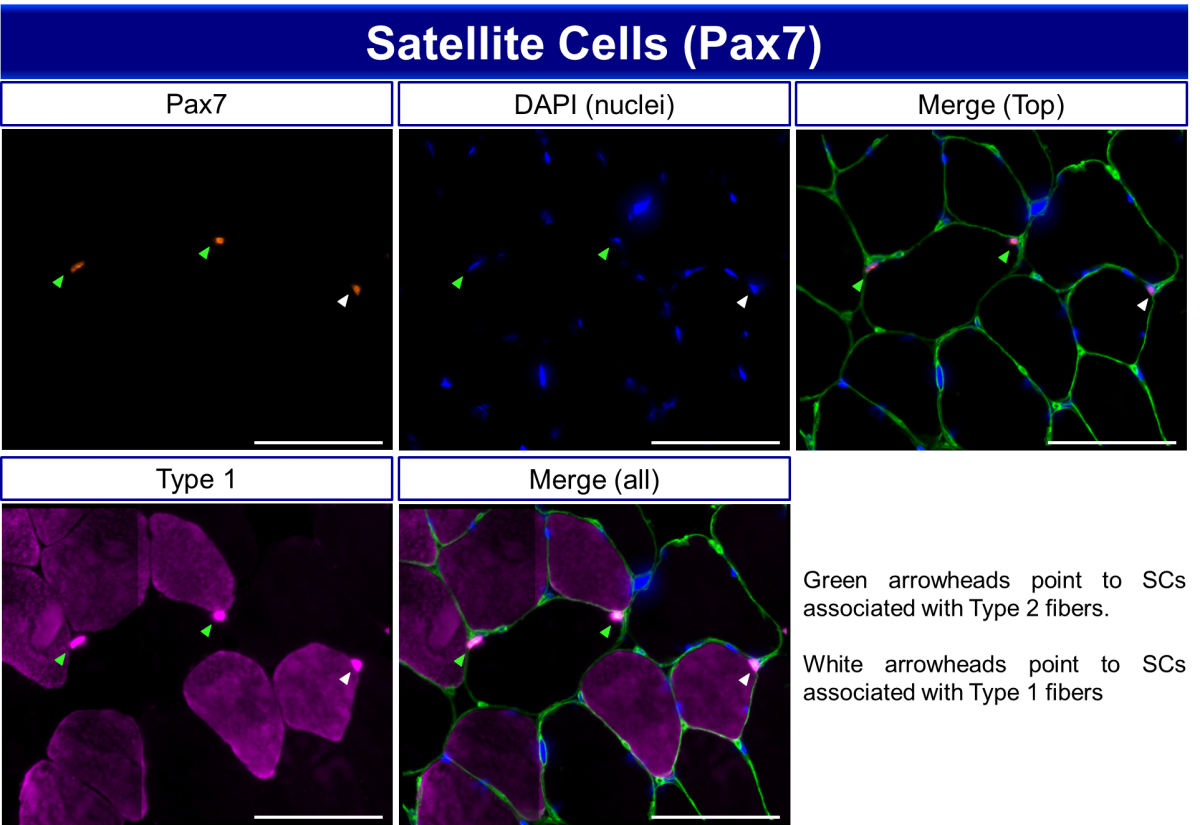
Immunohistochemical analysis of satellite cells (SCs, Pax7+) in human vastus lateralis muscle. Scale bar = 100 µm. Anti-laminin is used to delineate fiber borders and type 1 myosin heavy chain (MyHC) antibody to determine fiber type, so that satellite cells can be assigned to specific fiber types.
Fiber Type Specific Pax7 Staining on Human Muscle Cryosections
Fiber Type Specific Pax7 Staining on Mouse Muscle Cryosections
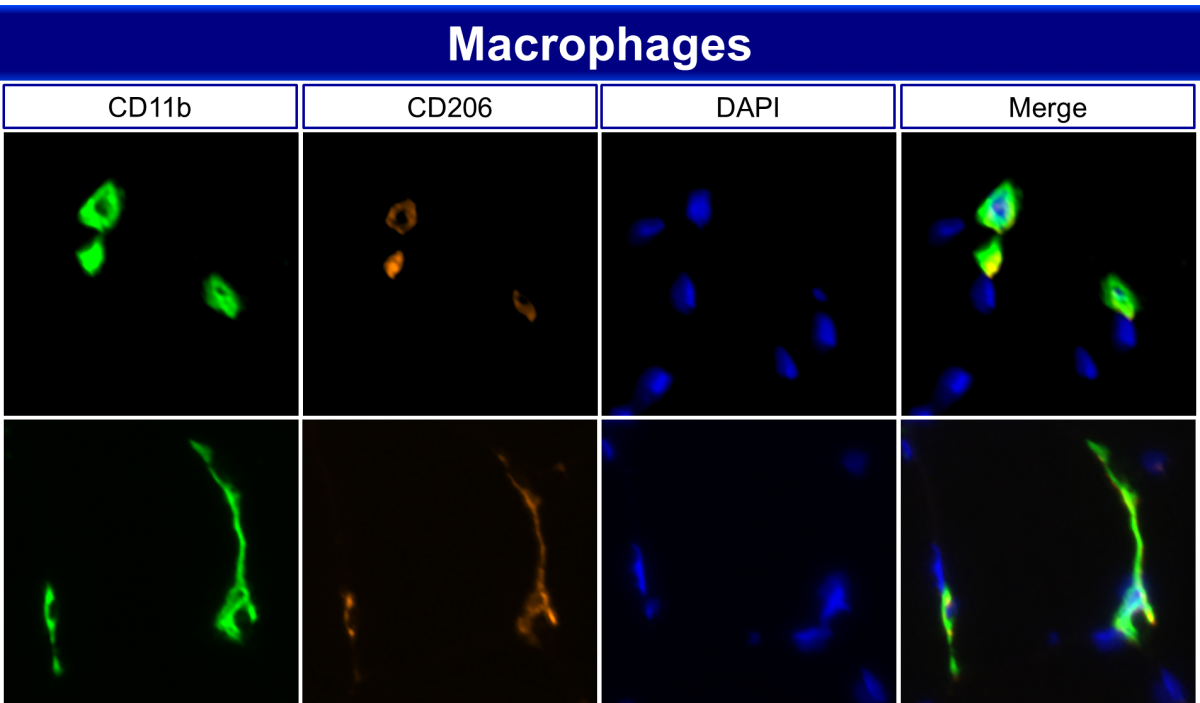
Immunohistochemistry demarcating M2 like macrophage populations in human gastrocnemius muscle. Skeletal muscle macrophages have known roles in muscle repair and regeneration. However, emerging evidence suggests that macrophage populations are also an important factor in muscle growth and maintenance, particularly in aging, obesity and disease.
Macrophage staining methods paper
Equipment
- MICROM HM525 Cryostat – user friendly, this cryostat can be set for temperatures between +5 and -35°C. Sections can be cut at thicknesses ranging from 1-500 μm. Completely motorized stage head automatically retracts and repositions for optimal sectioning. LED displays allows for tracking of wheel rotations or length of travel making it easier to track tissue mount usage. Equipped with Peltier element for rapid freezing of specimens.
- MICROM HM525NX Cryostat – the newer version of the HM525, this updated cryostat has completely digital, touchscreen controls allowing for more fine tuning of section thickness. Like the HM525, the 525NX has a cryobar equipped with a Peltier element which can cool to -60°C, used for rapid freezing of specimens.
- Zeiss AxioImager M1 Upright Fluorescent Microscope – with a semi-automated stage and filter wheel, the AxioImager can acquire whole section images (stitched). This scope is equipped with four fluorescent filters (DAPI, FITC, TRITC and Cy5) and 4 air objectives (5x, 10x, 20x and 40x) plus a 100x oil objective. Fluorescence is illuminated with an X-cite 120 lamp and liquid light guide. The color camera (AxioCam ICc5) acquires high resolution bright field images (5 megapixels), while fluorescence images are acquired with a high sensitivity black and white camera (AxioCam MRm). Image capture is done using Zen blue software from Zeiss.
- Zeiss AxioObserver D1 Inverted Fluorescent Microscope – the AxioObserver D1 is a fully manual scope outfitted with a color (AxioCam MR) and a black and white (AxioCam MRm) camera. Fluorescence is illuminated with an X-cite 120 lamp and liquid light guide. There are four available filters on this microscope: DAPI, FITC, Piston GFP (narrow band helps minimize autofluorescence) and TRITC. Available objectives include: 4x, 10x, 32x, 40x and 125x. Images are acquired with Axiovision software.
- Olympus BX61VS Upright Fluorescent Microscope – the Olympus BX61VS has a fully automated stage which can hold up to 5 slides at once. This microscope is great for imaging large batches of slides and can capture entire tissue sections and even entire slides. 2x, 10x, 20x and 40x air objectives are available for capture of overview images or high magnification mosaic images. The BX61VS is equipped with both a color (Pike F505, 5 megapixel) and a black and white (Olympus XM10) camera for bright field or fluorescent imaging. Four fluorescent filters are available for multichannel imaging (DAPI, FITC, TRITC and Cy5) and fluorescence is illuminated with an X-cite 120 lamp and liquid light guide. Images are acquired with VSI software.
- Independent Workstations with fully licensed versions of Zen blue – in addition to image capture, we have 3 independent workstations with fully licensed versions of Zen. These workstations can be scheduled for more complex analysis following image capture. One workstation includes a Microsoft Perceptive Pixel, 55” touch monitor, useful when outlining specific structures is necessary.
Equipment can be scheduled using our Sharepoint calendar (link provided following completion of training).
- ZEN Lite - ZEN lite allows you to open, view, change display, export image files acquired with Zen blue (.CZI). It also has some measurement and analysis features.
- OlyVIA - OlyVIA allows you to open and view image files acquired with the Olympus BX61VS microscope (.VSI).
- Image J - Free software provided by the NIH, Image J has several plug-ins that can open various file types for viewing, saving or analysis.
- Spectra Viewers/Analyzers - If you are unsure of which fluorescent labels to pair together, these viewers can help you make the best choice by showing you the excitation and emission spectra for multiple fluorophores within one display.